The simple formula of C1V1 = C2V2 is a lifesaver for bioscience researchers in the lab who are wanting to do dilutions. Here I will explain what the equation means and how you can use it.
What the equation means
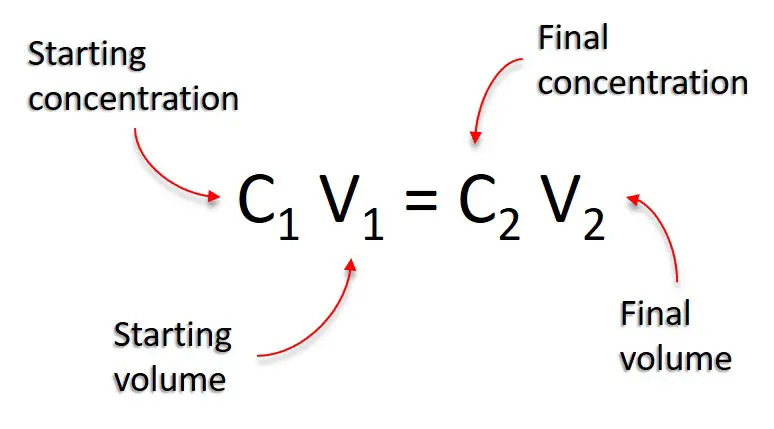 The equation has four components:
The equation has four components:
- C1 = Initial concentration of solution
- V1 = Initial volume of solution
- C2 = Final concentration of solution
- V2 = Final volume of solution
Put together, the equation translates to: the starting concentration multiplied by the starting volume is equal to the final concentration multiplied by the final volume.
Basically, if you have three of the four components of the equation then you can use these within the formula to calculate the unknown component. All you have to do is to rearrange the formula for your needs.
For example, if you want to calculate the final volume of a solution you would change the formula to:
Or, if you want to calculate the initial starting concentration of a solution you would use:
Once you understand the equation, it will become accustomed to your everyday lab work. Let’s take a look at some examples including numbers to help you understand this further.
Examples
- Calculate the amount of 10 μM forward primer solution to add to a PCR reaction (25 μL total reaction) to make a final concentration of 0.4 μM forward primer in the reaction.
So by using the C1V1 = C2V2 equation, we need to first rearrange this to work out V1 (the initial volume of primer we need to add). This would then make:
Next, we need to fill in what we know. We know the values for C2 (0.4), V2 (25) and C1 (10). So:
V1 = (0.4 x 25) / 10
V1 = 10 / 10
V1 = 1
Therefore, in this example, we would need to add 1 μL of 10 μM forward primer solution to a PCR reaction containing a total volume of 25 μL to achieve a final forward primer concentration of 0.4 μM.
- Calculate the amount of water you need to add to make a final concentration of 70% ethanol solution by using 100 mL of pure (100%) ethanol.
In this example, we are asked to calculate the final volume (V2). Therefore, the equation will look like:
We know the starting concentration (C1) of pure ethanol is 100%, the volume (V1) of pure ethanol we have is 100 mL and the final concentration (C2) we want to make is 70%. Putting this into the equation will look like:
V2 = (100 x 100) / 70
V2 = 10,000 / 70
V2 = 142.9
The final volume we need to make therefore is 142.9 mL. We know 100 mL of that is the 100% pure ethanol, so the volume of water must be 42.9 mL (142.9 – 100 = 42.9). So, adding 42.9 mL of water to 100 mL of 100% pure ethanol will achieve a final concentration of 70% ethanol.

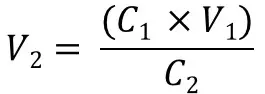
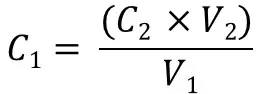
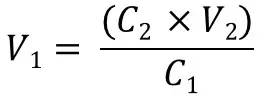



thank you so much
For example no. 1: would you add 1ul primer to 24ul reaction mix to make a final total of 25ul. Or, would you add 1ul to the 25ul mix (final total = 26ul)?
Hi Dominic,
So this question comes up a lot with students.
The correct answer is the first answer (add 1 uL of primer to 24 uL reaction mixture). This is because the FINAL volume will then be 25 uL. Don’t forget to factor in the volume of the primer (1 uL) as this volume when added to another volume will change the final concentration.
I hope that makes sense,
Steven
Thanks very much
THANKS DEAR…………
Thank you so so much finally I got it!!!
You are very welcome 🙂
Thank you so much..life saver ?
Thanks.This will help in my project work.
Thanks for your comment. I’m glad it helped you 🙂
Thanks steven! I have exam tomorrow and this article really helped! ???